[ad_1]
This technology offers a sustainable solution for everything from individual homes to large-scale industrial energy storage systems, bringing us closer to a greener future.
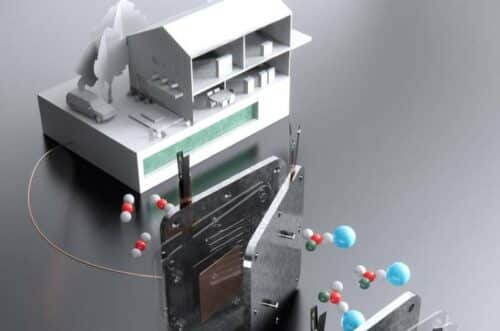
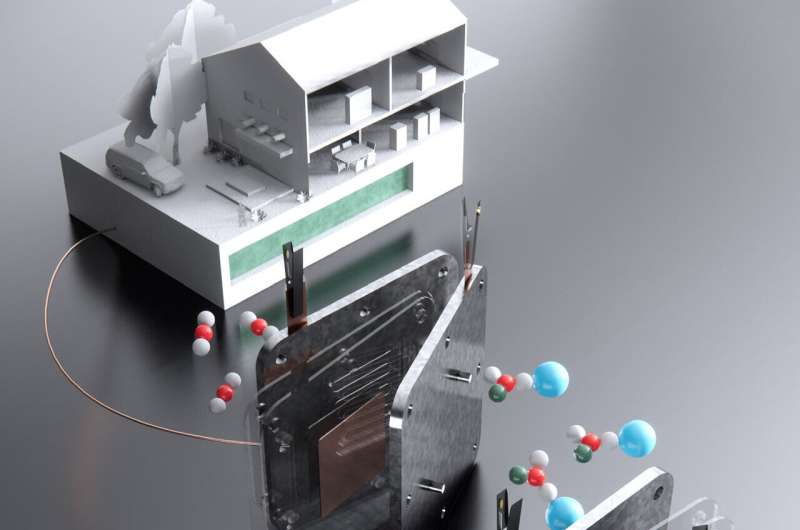
To combat climate change, researchers worldwide are tirelessly searching for innovative methods to extract carbon dioxide (CO2) from the atmosphere or industrial emissions and transform it into a valuable resource—the conversion of CO2 into a stable fuel that could replace fossil fuels in various applications. Most previous attempts at this conversion faced significant hurdles, including low carbon efficiency and producing toxic, flammable, or hard-to-handle fuels.
Scientists from MIT and Harvard University could revolutionise the field. They have developed an efficient process of converting CO2 into formate, a versatile substance that can be used like hydrogen or methanol to power fuel cells and generate electricity. Potassium or sodium formate, substances already manufactured at industrial scales and commonly employed as de-icers for roads and sidewalks, offer several advantages. They are non-toxic, nonflammable, easy to store, and can remain stable in ordinary steel tanks for extended periods, ranging from months to even years.
The researchers anticipate that it can be scaled up to provide emissions-free heat and power for individual homes, as well as industrial or grid-scale applications. Traditionally, CO2-to-fuel conversion processes involved a two-stage method, first converting the gas into calcium carbonate and then heating it to release carbon dioxide and convert it into a fuel feedstock like carbon monoxide. However, this second step was highly inefficient, typically achieving less than 20% conversion. In contrast, the new process attains a conversion rate of over 90% by initially converting CO2 into a liquid metal bicarbonate and then electrochemically transforming it into liquid potassium or sodium formate, using low-carbon electricity sources such as nuclear, wind, or solar power.
The resulting highly concentrated liquid formate solution can be quickly dried, for example, through solar evaporation, to yield a stable solid powder suitable for long-term storage in standard steel tanks. This process offers a significant advantage over hydrogen storage, which experiences leakage at about 1% per day. Methanol, another explored alternative for CO2 conversion, is toxic and unsuitable for applications where leakage could pose health risks. In contrast, formate is considered benign and complies with national safety standards.
The success of this process hinges on several key optimisations, including membrane materials, pH control, and the prevention of unwanted side reactions. A buffer layer of bicarbonate-enriched fibreglass wool helps mitigate side reactions, ensuring the system’s long-term efficiency. This groundbreaking technology opens up possibilities for diverse applications, ranging from household units to large-scale industrial or grid-based energy storage systems. Initial household implementations may involve a refrigerator-sized electrolyser unit to capture and convert CO2 into formate, which can then be stored for later use, providing power and heat as needed. In summary, this development represents a significant leap toward harnessing CO2 as a valuable resource, providing a viable path towards a sustainable future.
[ad_2]
Source link